 The Office of Prescription Drug Promotion (OPDP), once known as the Division for Drug Marketing, Advertisting and Communications (DDMAC) monitors company communications about medical products and when it deems those communications have gone outside regulatory parameters, the office issues a letter. For violations it regards as more serious Warning Letters are issued, for those deemed less so it comes in the form of an Untitled Letter.
The Office of Prescription Drug Promotion (OPDP), once known as the Division for Drug Marketing, Advertisting and Communications (DDMAC) monitors company communications about medical products and when it deems those communications have gone outside regulatory parameters, the office issues a letter. For violations it regards as more serious Warning Letters are issued, for those deemed less so it comes in the form of an Untitled Letter.
In past years, enforcement could be robust. In 1998, for example, 156 letters were issued to companies regarding communications. After declining the following year, in 2000 it took a real dip and by 2002 there were only 28 letters issued. While there was a slight uptick in 2009-10, the rate has continued to decline. The past few years saw 10, 9 and 11 for 2014, 2015 and 2016 respectively.
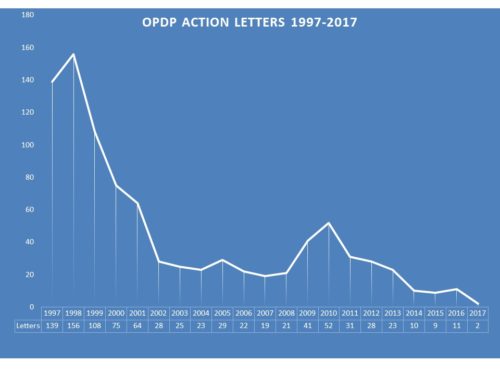 By comparison to this year, those are gushers. So far for 2017, OPDP has issued exactly two letters. One Untitled and one Warning Letter. Similarly OPDP had issued very few letters by this time last year (though more than just two) and then suddenly at the end of the year produced more than they had all year. So it is not yet certain that this year will see virtually no enforcement, but it is looking that way.
By comparison to this year, those are gushers. So far for 2017, OPDP has issued exactly two letters. One Untitled and one Warning Letter. Similarly OPDP had issued very few letters by this time last year (though more than just two) and then suddenly at the end of the year produced more than they had all year. So it is not yet certain that this year will see virtually no enforcement, but it is looking that way.
Why is it important? As noted many times in past postings on this subject, the letters issued by OPDP serve many purposes, one of which is to provide some insight into FDA’s thinking on current developments in communication in between the lengthy and arduous process of guidance-making. That is an especially relevant purpose in the context of today’s communications environment that changes quickly compared to the glacial process of policy enunciation by FDA. Case on point of course is the lengthy amount of time that has passed since FDA initiated a process to provide clarity related to social and digital media in 2009, saying guidance would come by 2010 and only providing partial guidance years later in 2014, with several questions still outstanding.Another notable trend over the past years has been that enforcement has tended to focus, though not exclusively, largely on companies that are smaller, less experienced companies in the marketplace.
And so the trickle of visible enforcement raises questions. Why has there been a drop in enforcement activity? Does it represent a shift in emphasis or new policy? An email sent twice to FDA on the subject went unanswered.
Perhaps this is a shift to focus enforcement into areas where there is greater risk – that would certainly make sense. In the past there have been letters issued about violations that might make some scratch their head and wonder about the real element of risk involved. But if that is so, it would certainly make even more sense if the agency were transparent about it and provided some insight into the thinking that is currently going on.
Does it mean that OPDP doesn’t care what is said in highly regulated speech? I would not count on it. They aren’t down to zero letters.
Some might be inclined to attribute the drop in enforcement to the change in the U.S. Administration, but the decline began long before. And who knows – perhaps we are still in for a December flurry of activity where several letters may be sent out in a matter of days. Until then, or until the agency chooses to provide some answers, we will be left to wonder.


Pingback: Why Has There Been a Drop in FDA Enforcement Activity? - PharmaLeaders.com