FDA has had a lot to say so far this year. A real lot. In fact, during the first three months of 2018, FDA issued 56 press releases, which is as many as they issued during the entire first half of 2017. It is also considerably more than were released during the first quarter of 2016. If it keeps up at this rate, it is likely that this will be FDA’s most prolific year ever for communicating with the public.
One of the many hallmarks of the era of Commissioner Gottlieb is the use of the “Statement from the Commissioner…” – as a vehicle to convey FDA developments. These statements deliver a more in-depth discussion than a regular press release. They also tend to highlight attention to a particular policy issue and also associate him very closely to the policy developments which are moving on a broad front to address a myriad of issues. During all of 2017, Commissioner Gottlieb issued 35 such statements, but in just the first quarter of this year, he has already done so 26 times (plus 2 others from other FDA officials) – which would annualize to over 100 for the year if they keep up at this rate. It is the volume of these statements that is accountable for the increase in volume of output in press releases.
In fact, as a vehicle, the Commissioner Statement has subsumed the role that press releases from the agency used to play. For example, FDA used to issue press releases about the issuance of new guidance documents. But this quarter, no press releases per se were issued, but rather guidance documents were announced by Commissioner Statements. So in the regular tally, you will now see the total number of such statements, but a subset of them will be parsed out by subject matter as if they were a press release for purposes of tracking subject matter. 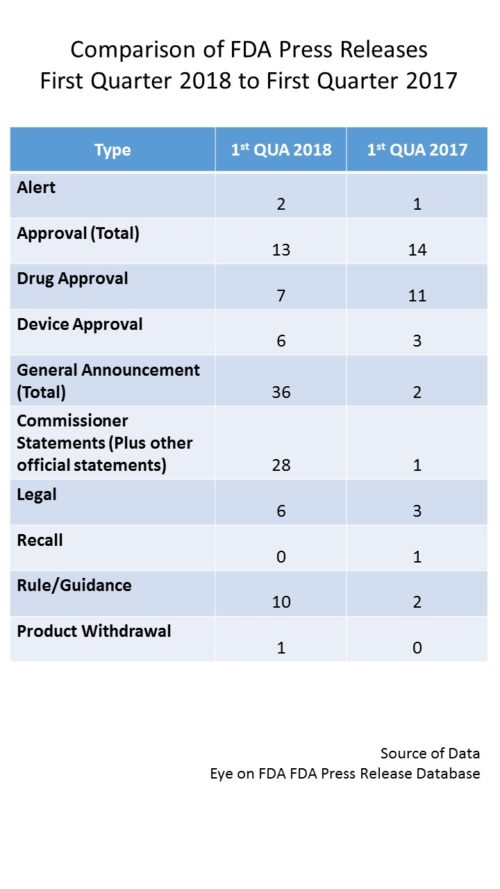
In addition to the bevy of Commissioner Statements, you can see that there have also been a higher number of rules, anticipated rules and guidances announced this first quarter, reflecting the policy push along a number of fronts – from nutrition and labeling to implementation of 21st Century Cures. We can probably continue to look for an increase volume for the balance of the year.
Otherwise, you can see that for approvals we are tracking with last year’s first quarter, though with a higher representation among devices.
But as noted in the beginning, this quarter is the culmination of change that has occurred in the agency – from a communications perspective and beyond. The press release is no longer the end – communicating a particular action of the agency – it has become a means to an end – to demonstrate momentum on the part of the agency overall. And combined with the prolific tweets of the new Commissioner (over 2800 from @SGottliebFDA so far), FDA has embarked on a new era of highly strategic communications.
An update to this report in early July.
Photo by G. Crescoli on Unsplash


