
Periodically it is a good idea to take the pulse of what FDA is saying. This is not by looking at each press release individually, though of course that is important, but rather to look at the aggregate of what is being said – and how does it compare to the past? Utilizing a database created to track and characterize the output by the agency, I did a look-back at the end of 2017. Time to do so again. There are some interesting results.
Undoubtedly, this has been a banner year for FDA in many respects. There were more approvals of new molecular entities by the agency than ever and more approvals of generics. Communications is no exception when it comes to volume. This past year, FDA issued a total of 289 releases, a healthy increase over the 164 of 2017 and the 122 of the year before that. In short, FDA has had more to say – a lot more.
More Approvals – Fewer Approval Announcements. Given that there were more new molecular entities approved than ever, you might thing that the increase in approvals led the increase in communications. It did not. In fact, the number of press releases about new approvals actually declined during 2018. There were 74 releases about new approvals in 2018 compared to 77 in 2018, with a smaller proportion of those (47) being about drugs or biologics compared to 2017 (52) – that despite the fact that more drugs were approved in 2018. So more approvals, fewer press releases on that front.
What was the driver of the huge increase? It was, after all, a 76 percent increase (by the way I had Wolfram Alpha do the math). The primary reason for the increase was the number of “Statements from the Commissioner”. Last year he sent out 36 statements. This year he increased it to 126.
What is he talking about? Everything it turns out. A good deal of his focus was the enunciation of policy change for the agency that included departures from the past and setting up new regulatory frameworks – from the use of animals in laboratory testing to modernizing the regulatory process for the development of targeted therapies to new steps in the implementation of FDA’s pre-cert program. And related, he sometimes announces the issuance of new guidance documents. and has focused on marking progress and milestones respecting some particular issues, notably various aspects of the opioid misuse epidemic and FDA steps taken to address it. But there were also clearly issues that rose to the top – those he cared about personally (opioids and e-cigarettes) and where he was perhaps demonstrating that the agency was taking some issues seriously and acting upon them – including
- e-cigarettes about which there were 4 statements over a two-month period;
- food safety – 4 statements about the Romaine lettuce safety issue from this year in addition to several more on food safety procedures in general;
- medical device regulatory approaches and approvals about which there were 6 statements;
- kratom – 3 statements about various actions around kratom, which is related to those he made about opioids;
- opioids – 11 more statements involving some aspect addressing opioid misuse and abuse – from new steps FDA was taking to new treatment approaches and much more
Certainly the Commissioner Statements allow the luxury to provide more context, background and reasoning than does a normal press release, and it was clear that there were a number of issues where he felt adding that context was helpful to explain to key stakeholders, most certainly including policy makers, not only why the agency is doing things, but seeking to underscore the announcement to demonstrate progress.
What Did FDA Talk About? When the communication wasn’t in the form of a Commissioner’s Statement, there were some definite increases in activity by the agency in some categories. For example, there were a much larger number of alerts about public health matters with 12 this year (romaine lettuce, e.g.) than last year with just 3. And there were a lot more announcements related to legal actions – seizures, warnings and the like this year (32) compared to last (19).
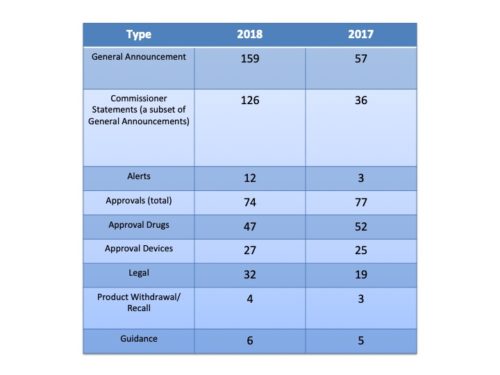
Note that the Guidance category does not include at least 5 Commissioner Statements that were in regard to new guidance documents the agency was issuing.
Much has changed at the agency over the past year and a half. There has been a concerted effort at policy change. But not to be overlooked, and a point made in a prior blog posting, is that communications is among those changes. It certainly seems worth keeping an eye on it.
Photo by AbsolutVision on Unsplash


Pingback: Recon: NICE Rejects Novartis' Migraine Drug Aimovig – Regulatory Focus | Everyday News Update