
In recent years, FDA’s Office of Prescription Drug Promotion (OPDP) has been diminished in the volume of its enforcement expressed through the issuance of Warning and Untitled Letters. OPDP now sends out only a handful of letters each year when it used to send dozens and dozens (see below for year by year breakdown). In fact, for 4 out of the last 5 years, the number of letters has registered in the single digits. The reasons for this drop have been the subject of speculation in the past. Perhaps compliance is way up. Perhaps it is a political decision. Or perhaps – more likely – it is a focus of resources. Rather than ferret out each and every violation it can find, significant or not, FDA is maybe looking to issue letters where it can make a point or make a difference.
Recent enforcement trends, such as they are, bear that out. In February the office issued a letter recently posted to the FDA site directed to a sponsor and principal investigator in relation to an investigational new drug. The agency took issue with a webpage where the investigational compound was the subject of claims and presentations that the agency said made conclusory representations when the safety and efficacy had not yet been established.
The letter follows two patterns out of OPDP. A feature of enforcement letters over the past few years is that they are predominantly directed at entities that are not household names – smaller pharmaceutical companies or in this case an imaging center (though last year did include letters to Pfizer and Eisai). But the second, and more significant characteristic is the violation that is being more often cited – for the promotion of an investigative compound.
In a review of the 330 letters issued by OPDP since 2004, a letter issued for the promotion of an unapproved drub has been the subject of only 18 letters – 5.5 percent of all letters. Those 330 letters covered over 1100 violations (letters typically cite more than one violation) which means that promotion of an investigative compound has comprised only 1.6 percent of all violations.
However, if you look at the proportion that this particular violation occupies in the past few years, it tells a different story. Fully half of all of the letters ever issued by OPDP involving promotion of an investigational compound have come out since 2016, comprising one quarter of all of the letters issued 2016-2019, or nearly 15 percent of all violations. Both numerically and as a proportion, actions regarding promoting an unapproved drug has risen substantially, signaling that this is a current enforcement priority for FDA’s OPDP. If you drop 2016 from the mix, the result is even more pronounced with one-third of the letters being about an investigational compound and comprising nearly one-fourth of all violations.
Risk information – either presenting it in a way that minimizes risk or under circumstances that omit risk information entirely – still is the most common violation, but clearly promotion of an investigational compound has the focus of the agency just now. So bear in mind, low enforcement does not mean no enforcement.
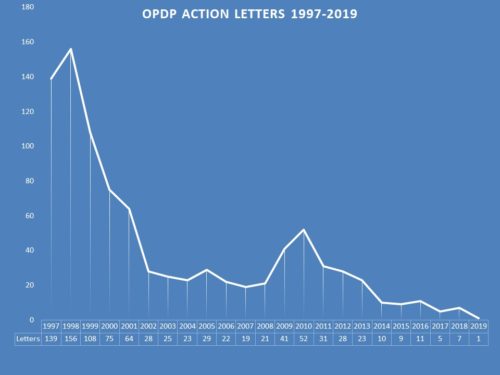
Photo by Goh Rhy Yan on Unsplash


Pingback: Pharma ad police issues 2019's first warning over promotion of unapproved imaging drug - WinWay Health Blog
Pingback: Pharma ad police issues 2019’s first warning over promotion of unapproved imaging drug – medicine-99.com