
One of the many things notable during the tenure of Dr. Scott Gottlieb as FDA Commissioner was the change in communications – a change marked not just by style but substance. In fact, in a March posting here, it was observed that he had set a new bar that shaded the agency with a more “activist” character – tackling big issues and communicating the agency’s outlook and progress on them – from teen vaping to opioids to drug pricing. He used communications to more thoroughly and more regularly to inform the general public, draw lines in sand to opponents, address concerns of critics and keep members of Congress in the loop. It was not just the tone and tenor of the communications that changed, it was the volume. In fact, the number of communications missives from the agency rose dramatically. In 2016, the number of FDA releases was 122; in 2017 it was 164 and in 2018 it was a whopping 289. The numbers alone speak for themselves.
He also used the platform of Commissioner to more personally communicate himself – in the persona of the Commissioner, rather than the agency. He did so by utilizing a heretofore little used mechanism called “Statement from the Commissioner” – a form of press release that had been rarely used prior to his tenure as Commissioner but used once, twice, thrice weekly once he assumed office and got in the groove. It put a personal stamp on agency actions and conveyed to audiences that he was on top of a number of issues. Commissioner Statements in 2016 numbered only 1, but in 2017 it was 37 and in 2018 was 127, or about 44 percent of all FDA communications.
Dr. Gottlieb left FDA in early April. How has that impacted FDA communications, if at all? Did the pace of Statements from the Commissioner slow down? Well, yes and no.
Comparing the first six months of 2016, 2017, 2018 and 2019, here is how the numbers roll. The number of press releases issued by FDA has been on the rise since 2016 and looking at the measure half way through the year (represented in blue) one can see that more releases (154) were issued in the first six months of the year than in any of the years since 2016.
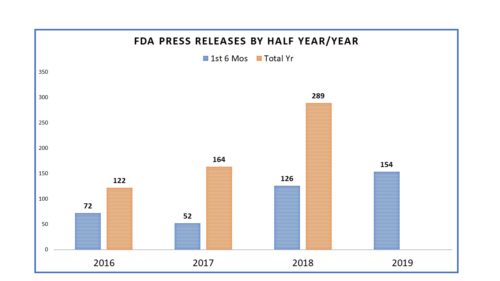
And as noted in prior postings on this topic, the number of Commissioner Statements, which are termed here as “Special Statements” certainly differed once Dr. Gottlieb became the commissioner. In 2016 Dr. Califf, Dr. Gottlieb’s predecessor, issued one. In 2017 he issued another. The rest that year were issued by Dr. Gottlieb and in 2018 one can see that the numbers were prolific.
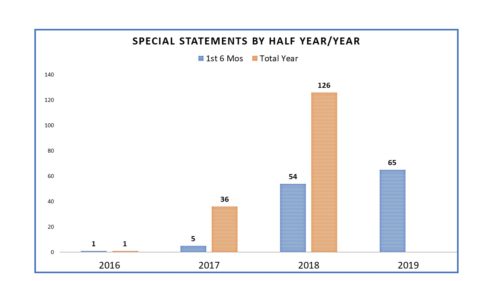
Which leads to one of the changes that has occurred since Dr. Gottlieb’s departure. Rather than have statements from the Commissioner’s Office, a number of Special Statements have been issued by Office Directors and other senior officials. In fact since Dr. Gottlieb’s departure, of the 65 Special Statements issued so far this year, several have been statements by senior officials, some of which were issued by Dr. Sharpless as Acting Director. In other words, the Special Statements have become more democratized, spreading laterally in the organization rather than being concentrated in the Office of the Commissioner. It is difficult to say what, if any, impact that has on the effectiveness of the communications or whether now they are less “special” and simply are statement – or how it may differ once there is no longer the word “Acting” preceding the word “Commissioner”. That said, FDA is still using them to draw attention to specific issues and the progress FDA is making on them, as the one issued yesterday from Acting Commissioner Sharpless – “Statement on the Agency’s Actions to Tackle theEpidemic of Youth Vaping and Court Ruling on the Application Submission for Certain Tobacco Products, Including e-Cigarettes“.
As to subject matter, here is a breakdown of this year so far:
- 9 Alerts on issues of public health
- 43 Approvals
- 79 “General Statements” which includes Special Statements
- 22 Legal Actions – seizures, warning letter announcements, etc
- 4 Rule or Guidance related statements
Perhaps the most notable aspect of all of this is that the number of missives from FDA has increased. If the six-month mark doubles for the year end, FDA will have issued more press releases than ever by my count. The agency has had more to say and is saying it more often (though July got off to a slow start with a huge gap in word from FDA from July 3 until July 15). We will revisit at year end to see how 2019 shaped up and what impact an appointment of Commissioner in the non-Acting capacity might have.
Photo by AbsolutVision on Unsplash

