The system of FDA Advisory Committees is in place so that when FDA needs advice – either on policy development, reviewing an issue with an already approved medicine, or regarding new drug approvals – the agency has a lot of expertise to consult. There are 31 advisory committees on hand organized by subject matter jurisdiction and expertise and 18 of them are under the category of Human Drugs. The Committees are comprised of a range of experts – from clinicians to practitioners to experts in clinical trial design and statistics. There is room for a patient and a representative from industry. They are summoned by the agency when considering a new drug or supplemental new drug application and generally meet about two months before the date set by the Prescription Drug User Fee Act (PDUFA) – also popularly known as the “PDUFA date”. They are not employed for use in every single approval decision, but in circumstances where the agency wants their input, usually the discretion of the division leader over the particular therapeutic category.
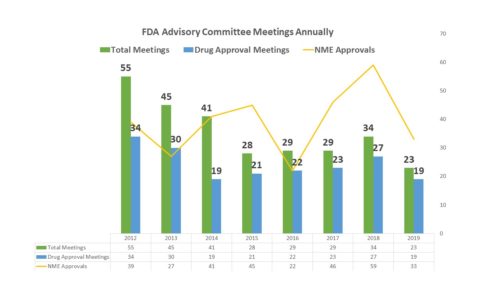
The agency has been asking for less advice than usual from the Human Drugs Advisory Committees – scheduling just 23 meetings this year, 19 of which were related to the approval of a new medicine . Granted, the year is not over, but given the organizational needs of holding such a meeting, FDA usually schedules them a few months in advance. There are unlikely to be any new ones scheduled for 2019, particularly given the holiday season will soon be upon us.
One possible reason for the need to consult with experts less frequently could be the emergence and uptake of Breakthrough Therapy status. This designation involves a more frequent and high level of consultation between the agency and the sponsor of a new drug application. Begun in 2012, the number of drugs that have been approved having had Breakthrough Therapy status has increased from 3 drugs in 2013 to 38 applications in 2018. The enhanced interaction between the drug sponsor and the agency may address many of the issues for which the agency would have otherwise sought consultation with experts.
Of course, another possibility is that there were fewer drugs to review. The number of NMEs approved so far this year is certainly less than it was last year. In 2016, there were only 22 NMEs approved, and 22 advisory committee meetings held that examined new drug applications. However in 2018 there were many more NMEs approved, but not many more advisory committee meetings considering NDAs for approval. This year, the number of NMEs has fallen, and so have the AdComms.
Whatever the cause, the notion that there are fewer AdComms may make lead some to feel there is less transparency to the process of approval. While they are long, and sometimes quite arduous to sit through, they are a setting in which one gets to see deliberation in action with respect to the approval process. You may not agree with everything, but you get to see it and hear it. Moreover, there is an opportunity for public input. There is a patient representative on each panel, as well as a non-voting industry representative. And perhaps most importantly in that regard, there is the Open Public Comment period during which patients, investigators and other interested stakeholders can share their outlook, experience and opinion and a docket is opened to receive comments. And it all happens before the media, who report on it in articles and on social media. Finally, if you miss it, there is both a transcript and a webcast. They are a complete learning experience for the observer, from providing insights into how FDA reviews safety and efficacy – how it develops concerns – what constitutes strength in clinical review and what spells weakness. We’ll have to see how next year shapes up.
And speaking of advisory committees, according to FDA’s current roster listings, there are currently vacancies on some of them.
- Arthritis Drugs Advisory Committee – 2 vacancies
- Bone, Reproductive and Urologic Drugs Advisory Committee – 2 vacancies
- Gastrointestinal Drugs Advisory Committee – 1 vacancy
- Non-prescription Drugs Advisory Committee – 1 vacancy
- Oncologic Drugs Advisory Committee – 1 vacancy
- Pharmacy Compounding Drugs Advisory Committee – 3 vacancies
Finally, at year-end, I’ll provide a look-back on how AdComms fared with respect to new medicines.

