Each year in January, it is relevant to look back on the activities of FDA during the course of the previous year and look at how it was the same or different from prior years, and to look at trends in new direction. This past year, needless to say was a doozy on all fronts. Let’s begin by looking at approvals.
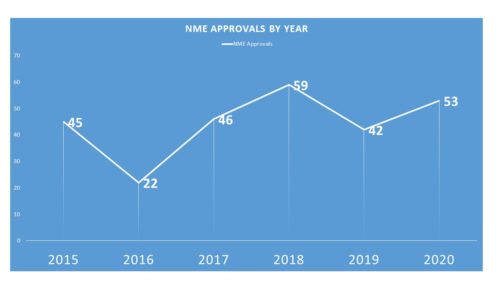
One might have surmised that the advent of the COVID-era might have thrown off new approvals and that NMEs approved would go diminish. That, however, was not really the case. During 2020, FDA was able to approve 53 – not surpassing the 2018 all time record of 59, but certainly an admirable second place.
One has to also take into account that the approval mechanism at FDA was particularly busy this year. The agency had a number of Emergency Use Authorization (EUA) applications to consider, many of which were for devices (tests) but some for therapeutics and two for vaccines that included the staging of two advisory committee meetings within a week of one another, resulting in the authorization of two mRNA vaccines – a platform never before commercially available. While not full licensure, the mechanisms for approval did utilize agency resources.
Notably, the 2020 NME approvals included 22 related to oncology, with 2 new GIST treatments, 3 in breast cancer and 2 approvals in prostate cancer. There were 2 new treatments for Ebola as well as an approval for a diagnostic in Alzheimer’s. And there were at least 14 approvals for conditions considered rare.
The number of FDA Advisory Committee meetings held for 2020 to consider new drug or biologic applications numbered 19, down from the previous year when there were 23 which was down from 2018 when there were 28. That may be less a reflection on the impact of COVID than it is the fact that so many investigational compounds come through the system with an enhanced status that may diminish the need for advisory committee input.
It was certainly reasonable on the part of many to expect that FDA’s capacity for keeping the pipeline moving through the regulatory process might have been negatively affected by the COVID-19 pandemic. Certainly the shift to virtual advisory committees must have been a challenge (and trying at times to listen to) as was the shift to remote work for employees, but in the end, the agency and the pipeline delivered the second highest number of NMEs to date, while some PDUFA dates have been missed or postponed.
Looking ahead, the interesting question for the year to come will be whether or not the pandemic will have affected the completion of clinical trials for new investigative compounds that were underway or recruiting during 2020 and what impact, if any, that will have on future numbers of approvals and NMEs.
Happy New Year, by the way.

