
Each year we look back to take stock of how the agency has been communicating in the course of the previous year and assess how that might compare to years gone by. In fact from year to year, there is a good deal of change that occurs. When Dr. Scott Gottlieb took the reins of FDA as Commissioner, the amount of communications material put out jumped enormously. And it did so again last year when the agency would have a lot to say about COVID-related activities.
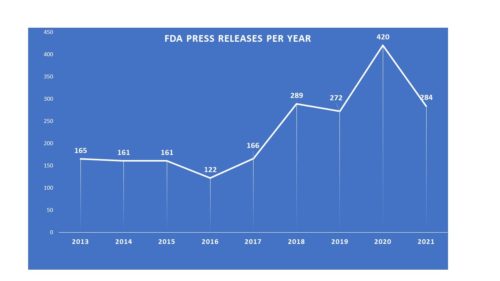
This year, however, not only was the agency putting out less, the number of releases this past year represents a somewhat inflated number. In prior years, the agency statements under a heading called “FDA in Brief“. These are statements that differ in format and nature from a traditional press release. They are more general announcements than they are news. Press Releases announce approvals, for example, while FDA in Brief statements can be used to “remind industry” or to announce that the agency will be holding a meeting. Press releases are often (though not always) released in English and Spanish, while FDA in Brief statements are not. These statements were housed on the FDA website separately from press releases. This year, however, the agency not only listed the FDA in Brief statements under their own heading, they also begin listing them in with the press releases they agency sends out. Since they did not do this in years gone by, that means that the number represented in the press release category is inflated. This year there were 43 “FDA in Brief” statements included in with press releases. If you actually removed them from the count, then the actual number of traditional press releases from FDA this year is 241, meaning the agency issued about 57 percent of the releases they issued during 2020.
Why the plunge? Certainly there was a good deal going on related to COVID, and the agency also approved a solid number of new molecular entities during, falling just a few shy of last year’s record number. One possibility may be the presence (or not) of a full time commissioner. The arrival of Dr. Gottlieb certainly saw an impact in volume. During nearly all of 2021, FDA has been under the direction of an acting commissioner.
In any case, here is what FDA talked about this year.
- COVID – 144 announcements were COVID-related in 2021, compared to 274 such announcements during 2020;
- Approvals – there were 77 announcements related to approvals this year, 22 of which were COVID-related announcements. In 2020 there were 40 COVID-related approval announcements. There were 43 drug and gene therapy approval announcements in 2021 (7 of which involved EUA announcements), 24 device approval announcements (7 of which were COVID-related) and 8 announcements related to Vaccines;
- Alerts – there were 9 Alerts issued by the agency this year, 4 of which were related to COVID – this compares to 16 Alerts issued in 2020, 7 of which were related to COVID;
- Legal – there were fewer releases related to legal issues in 2021, with only 12 issued regarding warnings issued by the agency or consent decrees compared to 21 in 2020;
- General Announcements – this category includes statements from the commissioner or from various division heads and here is where there the greatest numerical difference existed between 2020 and 2021. In 2020, there were 251 such pronouncements while in 2021 there were 156;
- Translation – Sometimes FDA communications are issued in both English and Spanish, sometimes not. There is no discernible rhyme or reason to it. There is also no indication why all communications are not translated. The translation rate appears roughly the same for the past two years – in 2021, 57 of the 284 releases (20 percent) were translated into Spanish, compared to 88 of the 420 last year (21 percent).
As far as what 2022 will bring, given the events of the past two years, it is hard to say. If the pipeline continues to produce in spite of the pandemic, there will be new approval announcements. There will certainly be new COVID developments. And the existence of so many products authorized under emergency use authorization is certainly an issue that will have to be dealt with. When will the products be fully approved? And while the imprint of the communications style of a new commissioner is something that might be anticipated, we are getting a commissioner who we had once before in 2016-2017, a period where the quantity of communications was not robust. And new in 2022, FDA has occasionally begun issuing something called “FDA Roundups” but have provided to date no new “FDA in Brief” statements. Something to keep an eye on.
Photo by AbsolutVision on Unsplash

