
Through its Human Drugs Advisory Committee process FDA has a vast array of outside experts to consult on matters related to product approval as well as questions about policy or safety issues concerning approved products. The mechanism provides an open and transparent process whereby people can see and hear the deliberations and have input during open public comment as well as through a docket established for that purpose.
But for those who have spent years watching – one thing has become clear. FDA Advisory Committees (AdComms) ain’t what they used to be.
First of all, they are all still virtual. At the beginning of the pandemic, FDA moved to staging virtual meetings. For anyone who had to suffer the onerous security ritual at FDA’s campus where the meetings were generally held that may be good news. In-person FDA AdComms were not really fun. The parking lot was light years from the entrance, the security screening was slow and laborious and once you were in, your eating options were quite limited. The switch to virtual meetings has meant that you can now watch and participate from the comfort of your own home – where presumably you can get whatever you want to eat. Ultimately, the virtual meetings certainly get the job done in terms of the exchange of information – even if you do have to put up with a lot of people needing to be reminded to unmute. But certainly the meetings have lost the personal touch lost in all virtual re-setting. You could see who showed up and who talked to who. I can recall a sitting U.S. Senator walking into a meeting to confer once. And there were many open public comment periods that were quite moving as a result of patient presentations in a way that they might not be in a virtual setting. The networking that used to occur – the informal interchanges – those are not happening. So that is one – albeit small – way in which AdComms have changed. But there are others.
Second, and perhaps more important, there have been fewer of them. In 2012 there were 35 meetings held by FDA for the purpose of considering new drug approval which is almost 3 meetings a month. By contrast in 2021 there were only 10 (see blue bars below in the chart tracking meetings from 2017 – 2022).
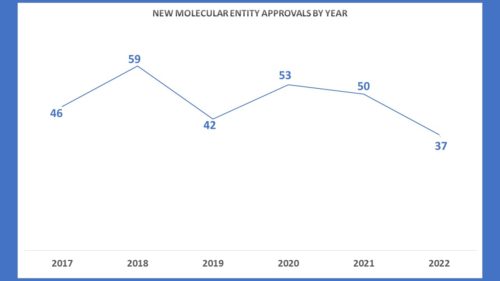
Yet in the midst of that range, the number of new molecular entities approved by FDA actually increased. Looking at the second chart below, one can see that the number of AdComms since 2018 peaked in the same year FDA approved a record number of new molecular entities (NMEs). But if you look at more recent history, you will see that during years of robust approvals for NMEs in 2020 and 2021, the number of AdComms went down considerably. That means that as FDA was approving more drugs of first impression it was consulting with outside experts through the transparent process of Advisory Committee meetings with less frequency.
As has been speculated here in the past, the drop-off may be a result – at least in part – of the rise of new pathways for drug approval such as Breakthrough Therapy Designation which promise enhanced access to FDA decision-makers, thereby perhaps diminishing the need for outside consultation.
During 2022, the number of AdComms held to consider new products rose a bit over the previous year to 19 scheduled meetings. However, with 4 of them cancelled, there were only 15 applications considered this year through the AdComm process. Pre-pandemic numbers were consistently higher. But conversely, in 2022 the number of NMEs plummeted.
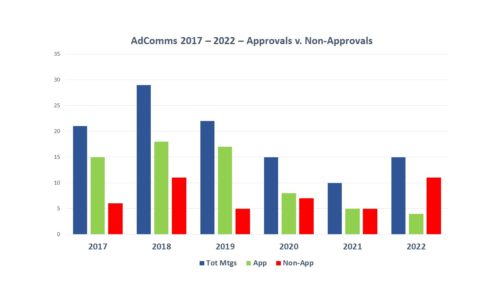
Third, AdComms aren’t as approving as they used to be. In terms of votes for recommendations of approval for new drugs, the track record was a little brutal this year. Of the 15 applications for new drug approvals in 2022 that went to AdComm consideration, the committees gave a thumbs up to only 4 (the green bars) – and in one of those FDA’s decision went against the approval recommendation of the committee and the sponsor received a Complete Response Letter. Moreover, of the 4 approval recommendations, none of them were unanimous votes in favor of the application. On the flip side – there were 11 votes on applications that failed to get a recommendation for approval (the red bars) and one of them was unanimous. In addition to the more negative tone as expressed by fewer approval recommendations, during 2022 there was an increase in meetings that were either cancelled or postponed.
So in sum, over the past few years, AdComms have been consulted less frequently while more NMEs were being approved. And when they were consulted, the outcome has been increasingly negative. Does the COVID-19 pandemic figure in here? It certainly seems that there is a difference between pre-and post- COVID patterns. Perhaps the virtual means for these meetings has an impact beyond the obvious in the deliberation. Or perhaps it is a coincidence. For those having an AdComm in 2023, we will watch and see what happens.

