Last week FDA’s Office of Prescription Drug Promotion issued its first regulatory action letter of the year. This was an Untitled Letter – a/k/a Notice of Violation Letter (NOV) – sent by the agency to Novartis in relation to promotional content regarding the cancer treatment KISQALI(r) (ribociclib). The communications vehicle that was the subject of the letter was a Direct-to-Consumer (DTC) television advertisement that had been submitted for agency review.
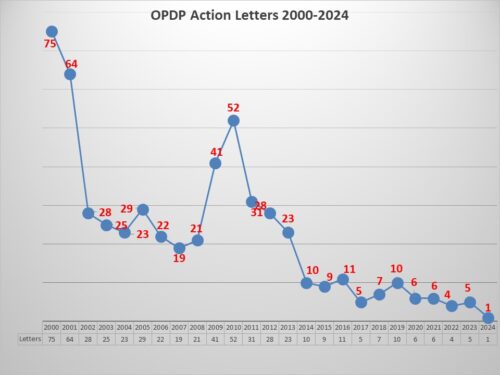
Before getting to the specifics of this particular letter, a look at how it stacks up against recent enforcement. Aside from being the first letter of 2024 – after 2023 when there were only 5 enforcement actions – the letter marks the second in a row concerning DTC TV ads, the last one being issued in October 2023. Too few to be a trend perhaps, but with such low levels of enforcement may nevertheless be noteworthy. DTC Ads, after all, do reach a very large audience.
There is a larger trend that may be in play – not the communications vehicle, but regarding the type of violation cited by OPDP. In this case, the most recent letter involved statements in relation to efficacy and the support cited for the making the claim. While the omission or minimization of risk is traditionally the most common violation cited in letters regarding promotional communications, the previous 8 letters issued by OPDP had issues with the presentation of efficacy information, making this the ninth in a row. Coupled with the fact that in 2023 OPDP announced that the office was conducting new research – “A Survey on Quantitative Claims in DTC Prescription Drug Advertising” and December 2023 guidance on “Presenting Quantitative Efficacy and Risk Information in DTC Promotional Labeling and Advertisements” it would be reasonable to assume that the presentation of efficacy claims is a subject getting the particular attention of the agency.
Regarding this particular letter, in addition to the characteristics already mentioned, the claims that were the subject of OPDP action involved expressions regarding quality of life (QoL). Specifically, the agency cited claims that the drug “helps preserve quality of live so that you’re not just living, you’re living well.” The claim was accompanied by a cited reference describing a clinical trial where QoL was included as a secondary endpoint.
FDA took issue with several aspects of this claim. A few of the more interesting ones – (1) the agency said that the cited study design precluded drawing conclusions regarding QoL and that the outcome could have occurred by chance alone. Even though OPDP noted that the ad carried a SUPER on a frame that stated that the analysis was not pre-planned to detect a false positive that it was insufficient to address the potential for creating a misleading impression. The agency said that the data from the study, while hypothesis generating, were not conclusive. (2) Moreover the collection of QoL data, the agency said, did not account for confounding factors that might be present for individuals from non-cancer related factors such as other health problems or personal life-issues. (3) In addition, the agency took exception to the claim that treatment with the medication “helps preserve quality of life so… [patients] are living well” because OPDP said that this presupposes that the patients were living well at the outset of the clinical trial, and (4) A voice over and graphic in the ad claimed that patients would “live longer” which is explained further in a SUPER, but the agency found that this presentation was in competition with distractions that undermined audience understanding of overall survival, with the agency citing data on audience reading comprehension. The agency found the expression of claims was out of balance with the qualifiers to those claims. A thorough reading of the letter will yield further insights into the OPDP perspective and will give a more fully rounded and detailed understanding of the agency’s thinking in this regard.
In sum, this Untitled Letter signals not only a continued interest in the subject of how companies express efficacy data in promotions generally, but specifically sheds a light on the guard rails the agency has in mind relating to claims regarding quality of life.

