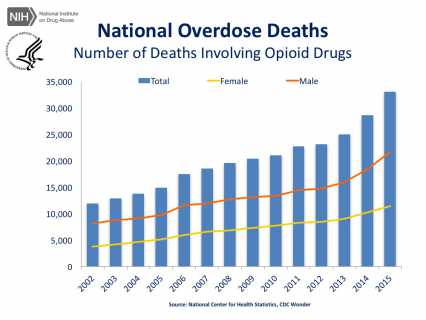
With deaths rising each year, there is no question of the urgency that something needs to be done to address the opioid misuse epidemic in the United States. And in addition to mortality there are the lives touched by these deaths, splintering relationships and families, incurring enormous personal and financial cost.
 During his confirmation hearings, the opioid issue was a primary concern expressed among policy makers and Commissioner Gottlieb provided reassurance that it for him a top priority. The Strategic Policy Roadmap released by FDA in January has as its first stated area of concentration to reduce the burden of addiction crises.
During his confirmation hearings, the opioid issue was a primary concern expressed among policy makers and Commissioner Gottlieb provided reassurance that it for him a top priority. The Strategic Policy Roadmap released by FDA in January has as its first stated area of concentration to reduce the burden of addiction crises.
What the Strategic Policy Roadmap Lays Out and What It Might Mean- Addiction is naturally broader than the opioid epidemic, including nicotine addiction as well. The Roadmap begins by addressing opioids but also addresses nicotine related goals. Below are key principles derived from the document issued by FDA followed by notes on what has already been done by the agency under each category as well as thoughts about what might be expected.
- People are inappropriately prescribed opioid drugs
- Early last year, FDA issued warnings about use of codiene and tramadol in children and nursing mothers and in 2018 acted to revise labeling regarding use of cough medicines containing codeine or hydrocodone in persons under age 18;
- Also this past year, FDA made revisions to the FDA Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics;
- Look for ways to carve out specific circumstances whereby other types of pain relief be first attempted prior to introducing opioids;
- Look for possible additional modifications to the FDA Blueprint;
- Look for focused attention on facilitating non-opioid pain options.
- Durations of prescriptions are often out of sync with need
- In a special statement issued this month regarding OTC loperamide, Dr. Gottlieb discussed the use of blister packs to limit exposure in an OTC product and it is not unlikely that such packaging – and limited courses of treatment – be examined for use when there are circumstances where the pain being addressed is short term;
- Look for the potential development of short-term use packaging options, such as blister packs where the number of pills dispensed is limited and exploration of any other means to limit the number of pills for specific circumstances. This echoed similar statements about packaging made in October 2017.
- Conversion of market to use of formulations with deterrence
- In late 2017, FDA issued a guidance doc meant to encourage the development of generic opioid medications with deterrent properties;
- In addition to enhancing entry of deterrent formulations may be shunning those without deterrence away from the market. In the February 12 Federal Register, FDA published a notice that it was not approving an instant release opioid formulation that lacked deterrent properties.
- More options are needed to address addiction
- First off, FDA specifically states the need to approve more treatment options for addiction and in fact, in November the agency approved the first device with an indication for treating withdrawal symptoms;
- In addition, in late September 2017 FDA provided an alert to medical providers on minimizing risks associated with medically assisted treatment (MAT) for patients seeking help;
- Finally late last year, FDA approved the first monthly injectable (MAT) for opioid use disorder having given the application both Priority Review and Fast Track designations;
- Look for a possible education effort to reduce stigma. Part of the Roadmap references the role that stigma might play in reducing access to therapy. This signals possible plans to address the issue through a formalized education effort, perhaps in conjunction with other federal agencies.
- Addressing Nicotine Addiction in Combustible Cigarettes
- Here the Roadmap states that FDA is developing a comprehensive approach to regulating nicotine by regulating nicotine levels to make smoking less addictive. This is one area of the document with very few details on the actual route to achieving this goal. However, in December, FDA did launch a new campaign aimed at smokers called “Every Try Counts” as a print and ad campaign to support those who have tried and failed to quit within the past year.
- Helping More Smokers Quit and Stay Quit
- The plan states that FDA will take a fresh look at products that can deliver nicotine without burning tobacco. However, a recent advisory committee meeting reviewing a heated (not burning) tobacco product resulted not only in a sobering vote by the committee against recommending the product claims, but a letter from 10 U.S. Senators urging FDA not to approve the products in question.
- Look for a series of guidance documents and rules to implement a new framework on nicotine and for the encouragement of medicinal nicotine products that may support people in their efforts to quit smoking.
That’s it for the first arm of the plan. In the next installment, we will take a look at access issues and public health.
Photo by Nadine Shaabana on Unsplash


