At the mid-year point, I usually do a check-in to see how we are doing on new drug approvals and how it compares to years gone by. I am quite tardy checking in on that front, so let’s get it done.
To set a little context, last year (2017) with 46 approvals of new molecular entities (NMEs) at FDA, it was a record year. However, it followed a year (2016) that was a little on the slow side with only 22 which, in turn, had followed another record year in (2015) with 45 approvals. So one year up, one year down, one year up.
Everyone is always interested in the number of new drugs being approved at FDA, but this year may be of particular interest because it comes in the wake of a broad policy push to lower the regulatory burden and to improve the pathway to support innovation. That is manifest not only in the passage and implementation of the 21st Century Cures Act, but in the repeated statements by the new Commissioner aiming at “modernizing” the approval process without sacrificing the agency’s gold standard for safety. It will be some time before that can be fully assessed, but in the meantime, many mechanisms have been put into place – such as Breakthrough Therapy designation, to enhance the regulatory process. One of our first insights into the effectiveness of these efforts is with the rate of new approvals for NMEs.
So at the first half of the year, things are coming along and we do not appear to be having the kind of dip we saw in 2016. In fact, FDA is at a slightly lower level of approvals than the previous year, but still going at a good clip. As of June 30, 2017, FDA stood at 23 approvals of new molecular entities, comprising exactly half of what would be the total for the year – 46. As of June 30, 2018, the number stood at 20, only two less than were approved the entire year of 2016. 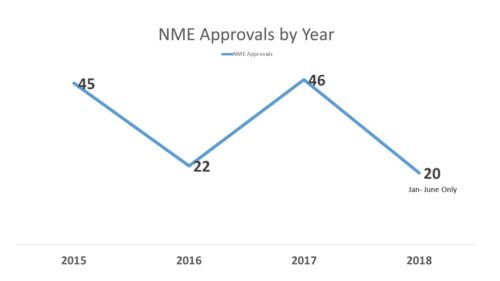
The number so far is high and if we keep the pace, the year should reflect a healthy amount of approvals. Will we do it? A few “surrogate endpoints” to look at to see how we are doing.
First, are there many advisory committees scheduled to hear drug NDAs and are they for NME? On that front, it does not look so hot right now – the advisory committee schedule page does not list any adcomms for drugs right now. Any that come much later in the year would likely represent PDUFA dates that will fall in 2019.
The other place to look is at known PDUFA dates. I run a little database on PDUFA dates gleaned from public sources such as company press releases. For the balance of the year, I have 42 PDUFA dates listed, but only 24 of them are for NDAs, as opposed to applications that are supplemental. That is not likely the entire universe of dates between now and the end of the year, only the ones that are apparent to me. Therefore the number is likely larger.
One final note given my lag in timing – since the mid-year mark, we have seen a healthy number of approvals. So in the end, we are likely to have a year that is on par, if not exceeding, last year’s number of NME approvals. Whether that is the effect of a healthy pipeline, the enhanced policy efforts in regulating the approval process or a combination of both still remains to be seen.


