
During the past few years, there has been a renewed focus on facilitating the development of new medicines by providing new mechanisms to streamline the review process and get drugs to patients more quickly. The passage of the 21st Century Cures Act and its implementation by the Food and Drug Administration has aimed, among other things, to speed innovation with the aim to bring more treatments to patients more quickly without compromising FDA’s safety standards.
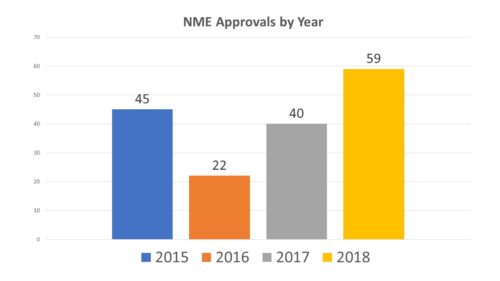
Has it worked? At first blush, there has been tremendous output in terms of new drug product approvals. In 2015, there were a record 45 approvals of new molecular entities, followed by a dip in 2016 to 22 (the 21st Century Cures Act was signed into law in 2016). In 2017 it went back up to 40 and last year we saw a whopping 59 approvals.
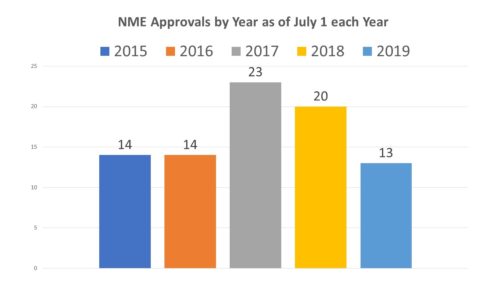
But so far 2019 is not shaping up. After the half of the year, we are only at 13 approvals, or on average a little over 2 a month, compared to the 2018 when there were 20 approvals by July 1.
How does mid year 2019 compare to other mid-year tallies? Actually not so good. While it would appear to be lagging – certainly far behind mid-year of the past two years – it is actually not far behind 2015 which was a record-setter by the end of the year, indicating that a mid-year assessment is not always a very crystal ball regarding the rest of the year. In 2016, for example, there were 14 approvals at mid-year, but only 8 over the following 6 months. The year before there were also 14 at mid-year, followed by 31 during the last half of the year. So one might think that there is at least a chance this year will pan out.
But, looking at a couple of indicators might dampen the spirits.
Advisory Committee Meetings – AdComms are down. The first indicator of how we will fare over the next six months is a near-term one and not a wholly practical one. Advisory Committees are not scheduled for every new drug approval. But it is still a factor and right now, it is one that is running low if you consider how many advisory committee meetings are scheduled as of now to consider new NDAs. These meetings are usually scheduled about 6 weeks out from the date of the meeting. Currently, there are only five advisory committee meetings scheduled to discuss drug approvals in the coming months, and one of those is an sNDA. One other point about advisory committee meetings, FDA has only held 9 so far this year to consider new products. By this time last year, the agency had held 16.
PDUFA Dates – That leaves us with PDUFA dates – certainly the more informative indicator. PDUFA dates are the date by which FDA will announce a decision about a new drug application, which may or may not be a new molecular entity. PDUFA dates are generally proprietary and there is no way to absolutely know all of them if companies do not choose to divulge the exact date. That said, many companies either do explicitly state their PDUFA date or at least give a ballpark idea by the date that specific announcements are made by press release.
Tracking PDUFA dates from year to year then is an inexact effort. That said, by my count there are a total of 108 PDUFA dates for the entire year of 2019, compared to 119 for 2018. Of the 119 in 2018, at least 78 were for NDAs (as opposed to sNDAs), meaning that this number could include NMEs, compared to a total of 61 NDA-related PDUFA dates in 2019. Of the 61 NDAs PDUFAs for 2019, 29 of them have dates that have passed, leaving only 32 possibilities. And finally, if all 32 were NMEs, which is not the case, that would still leave us short of last year when combined with the 13 approvals during the first half of 2019. By contrast in 2018 by mid-year, there were still 44 PDUFA dates left.
All in all, if you are looking for a banner year, this may not to be it. And if the numbers remain low, it may call into question for the true impact of the 21st Century Cures Act. Time, and transparency, will tell.
Photo by freestocks.org on Unsplash

