
As the year began, no one really thought that within a few months, the systems upon which we rely on for just about everything would be so severely disrupted and tested by the presence of something we cannot even see with the naked eye. But of course, that is what happened. By March we were remaining at home, including people who worked at FDA who assess new drugs for approval. What would the impact be on the approval process?
In response to written questions submitted in March, FDA indicated that the situation was fluid and could not say with certainty whether new approvals might be delayed, and also indicated that the agency was considering virtual advisory committee meetings. In April the agency reinforced the potential for disruption when it issued a statement saying that while it was meeting its PDUFA date obligations, it was uncertain that the agency could sustain its current work performance given the demands of the pandemic.
Now after six months of the COVID-19 pandemic, one might have expected that the approval of new medicines by the Food and Drug Administration might have been negatively impacted as a result. In fact, did everything that was going on – or more to the point not going on – did all that make a dent?
Looking to the approvals of new molecular entities, it appears not. In fact, so far, 2020 is doing rather well. Below is a chart comparing each year since 2015 at the 6-month mark of the hear. Not only is 2020 doing well, it is doing better by mid-year mark than any of the previous 5 years.
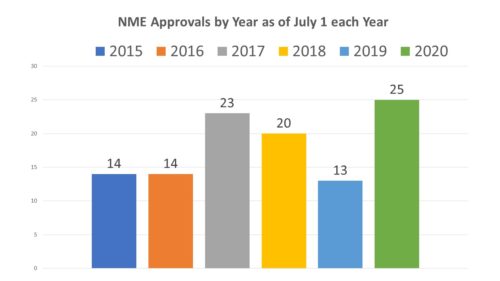
Mid-year comparisons are not always telling. For example, in 2018, there were only 20 approvals as of June 30, behind the number of the previous year when there were 23, but by year end new approvals in 2018 set a record at 59, far outpacing the previous year.
Another factor that might have adversely impacted the number of approvals for this year (though to a far lesser degree) are the number of FDA Advisory Committee meetings. Last year by June 30, FDA had held 9 meetings to consider new approvals. This year there were meetings either postponed to a date uncertain or cancelled altogether, while a few have been held virtually. During the first half of 2020, FDA AdComms were scheduled to discuss 8 NDAs, BLAs or sBLAs.
As far as upcoming PDUFA dates, I have fewer on my list for the second half of this year than I had last year at 45 NDAs or BLAs, at least 11 of which are Priority Review. In any case, with the number of approvals during the first half of the year, it suggests that this year will be at least on par with prior years.
While approvals are going along at a good clip during the first half despite COVID-19 challenges, this is occurring in an environment where patients are consuming fewer health services. That includes prescription medicines fueled in part by the fact that physician/patient interactions declined. Either because they have lost insurance and therefore their access, or because they feel unsafe in a medical care setting out of fear of COVID-19, patients are making fewer visits, getting less care, and engaging in diagnostics less. In the end, that leaves this a good news/bad news proposition. In spite of the challenges posed by the shutdown and distancing, the agency is nevertheless still approving NMEs at a healthy pace. But conditions in the greater environment mean that launch and uptake of these newly approved medicines will not be as robust as they might have been under different circumstances.
Photo by Sincerely Media on Unsplash

