
FDA’s Office of Prescription Drug Promotion (OPDP) issued the first regulatory action letter for 2022. This one has some notable characteristics.
As has been frequently noted, enforcement actions by OPDP have diminished considerably over the years. Another characteristic of recent enforcement actions over the past several years is that smaller, less well-known companies have mostly been the recipients of letters from OPDP. That said, the last two letters – the final one of 2021 and the first of 2022 both went to the same company – Lilly. Here are a few other facts to put this letter into context:
- This one, as was the last one, is an Untitled Letter, considered the less serious of the two types of letters sent out by OPDP;
- The product involved was one for diabetes, one of 7 letters issued since 2003 by OPDP regarding a diabetes product covering 10 different communications vehicles (in 2009 GSK received a letter covering 3 sponsored links for separate products);
- The communications vehicle that was the subject of this letter was an Instagram Post, one of two social media violations related to diabetes over the past several years (the first was a Facebook posting for Mannheim’s Affrezza);
- It is the second time FDA has taken action in relation to an Instagram posting, the first having been issued in 2015 regarding a product for morning sickness.
So why did OPDP act upon this Instagram post? According to the letter, the agency noted first of all that the video that was part of the Instagram post stated that Trulicity could “lower A1C along with diet and exercise” but that the Medication Guide specifically states that the drug is used for people with type 2 diabetes. There were additional limitations to the use of the drug, including that it is not recommended for people with severe stomach or intestinal problems, nor should it be used in people with type 1 diabetes. Therefore, the agency said, the indication was not adequately communicated. Moreover, when it came to risk information, OPDP noted that the benefits of Trulicity were emphasized through “attention-grabbing” and fast-paced video, but the the risk information was presented was included in a small window that was difficult to read and therefore presented a challenge in comprehension. In addition, one of the side effects regarding hypoglycemia that was in the Medication Guide did not appear in the posting, leaving the overall presentation minimizing risk associated with use. FDA noted that the product has a boxed warning and that there have been past advisory communications from FDA have expressed concerns.
It is perhaps worth noting that while this violation involved a communication via a social media platform, the issues addressed in the letter were not because it was a social media platform. Essentially, OPDP said, a video lacked balance and omitted some material issues. It was not the medium, it was the message.
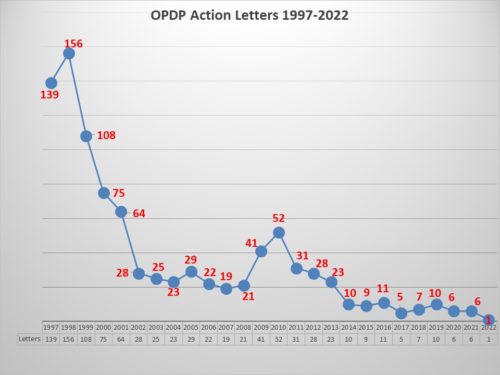
As you may recall from recent postings, last year FDA decided to remove Warning Letters, considered a more serious infraction, from the list of OPDP regulatory actions. The above chart portrays a combination of Untitled Letters and Warning Letters. However, FDA now lists Warning Letters in a searchable part of the FDA website that lists all Warning Letters issued by any part of the agency, while the OPDP portion of the website now only lists Untitled Letters. This means that in order to get an understanding of all the actions taken by OPDP, you now must consult two different parts of the FDA website. Confused? Well, yes, it is in confusing. Warning Letters can be found performing a search here, while Untitled Letters are listed here.
Eye on FDA maintains a database that contains the results of Warning and Untitled letters issued by OPDP going back through 2003. It includes 352 letters covering over 450 communications vehicles and over 1100 violations. The database tracks disease area, type of communications vehicle, whether or not the product had a boxed warning, whether or not it involved a Warning or Untitled Letter, whether it was a digital or traditional communications property and of course, the violations cited in the letter.
Note: In a prior posting reviewing 2021 regulatory actions, it was stated that FDA issued 5 regulatory action letters for the year. Since that writing, FDA posted another letter in mid-January that was sent to a company on December 19. That posting has been updated to reflect the late addition from FDA.
Photo by Goy Rhy Yan on Unsplash

