
For those working closely with the development of new medicines for FDA approval, it can be informative respecting the future to look back at recent activity and take note of any potential changes from years past. Now, with no more AdComms scheduled for 2023 – and it being highly unlikely that any will be – it is that time of year to do just that. What we find is that it was somewhat of a milestone year in terms of volume, outcomes and style.
Virtual Meetings. Let’s start with the obvious. We are still in virtual meeting territory. At the outset of the COVID-19 pandemic, FDA began conducting advisory committee meetings virtually. Prior they had been held in-person on the FDA campus (and prior to that, in hotels around the Washington, D.C. area). The quality of the virtual meetings has gotten better over time, though there are still occasional technical issues that impact the quality of the proceedings. Lost also are the many collateral benefits to in-person meetings where there is an ability to network among attendees and media, among other things. While 2023 maintained virtual meetings, it is perhaps noteworthy that FDA has scheduled an in-person advisory committee meeting of the General Hospital and Personal Use Devices Panel of the Medical Devices Advisory Committee to be held February 6, 2024. Does this signal a return in 2024 to in-person meetings? In response to outreach, the media office only would state that that “[i]n person and hybrid meetings will continue and will depend on the particular meeting and committee.”
Volume of Meetings and Outcomes. During 2023, there were a total of 29 FDA Advisory Committee meetings related to human drugs, 25 of which were held to discuss either the approval of new drugs or the approval status of medicines approved under the accelerated approval pathway. Of those 25 AdComms, votes were held during 23 meetings, 18 of which had votes recommending approval and 5 of which did not. There were two examples of meetings where advisory committees recommended approval, but ultimately the agency did not approve – and there were no circumstances this year where the agency approved in spite of a lack of such recommendation from a committee.
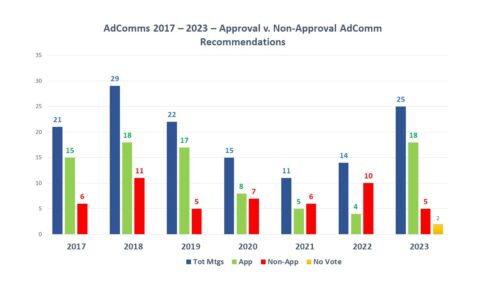
There are two things of note from this year. First, there was an increase in the number of meetings held to consider the status of new medicines, the most since 2018 and far more than were held in 2020, 2021 and 2022. Secondly, the meeting outcomes were much more positive than they were in those same years. In fact, in 2021 and 2022 more drug applications resulted in negative recommendations than positive ones – with the number of negative outcomes in 2022 being more than twice positive recommendations. By contrast, in 2023, the number of recommendations for approval was over three times the number of negative outcomes. Both in terms of volume and outcomes, the needle has moved considerably.
Committees with the Most Action. No surprise – the committee that met the most times was the Oncologic Drugs Advisory Committee, with six meetings during 2023. The Antimicrobial and the Cellular, Tissue, and Gene Therapy each met three times. Those that met twice included the Anesthetic, Endocrinologic, Peripheral and CNS and Pulmonary committees. Those that met only once included the Cardiovascular, Gastrointestinal, Obstetrics and Psychopharmacologic.
About Voting. Much was made earlier in the year when FDA Commissioner Robert Califf stated that he hoped to see more discussion and less voting during advisory committee meetings. Last year, FDA experienced a great deal of public attention on the fact that the agency approved a treatment for Alzheimer’s after a lopsided negative vote from the advisory committee meeting on the matter. At the same time, the head of the Oncology Division at FDA was quoted in media as saying that the role of voting was very important. Things do not change quickly at FDA as a rule, and this year we did see two advisory committee meetings where there was no voting question posed to the committee. The first was a meeting of the Cellular, Tissue, and Gene Therapies Advisory Committee meeting to discuss an investigational treatment for Sickle Cell Disease, and the other was a meeting of the Oncologic Drugs Advisory Committee which was convened to discuss updates on the accelerated approval status of two new drug applications previously approved under the accelerated approval pathway. We will have to look to 2024 to assess whether there is any meaningful change in FDA policy in regard to voting.
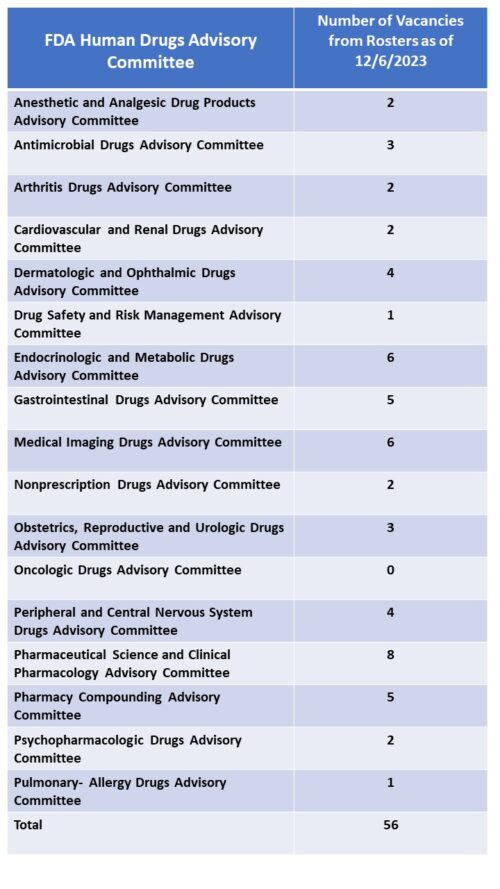
Committee Vacancies. A survey of the rosters of all of the human drugs advisory committees shows that the agency is ending the year with a large number of vacancies with 16 of the 17 committees with vacancies. In total among all the committees, there are 56 vacancies.
Things to look for in 2024. (1) Voting questions for meetings considering NDA approvals; (2) getting these many vacancies filled; (3) whether or not there will be in-person meetings to put that meeting room to use again; (4) whether the number of meetings will hold steady and (5) will outcomes continue to be so positive for new drugs?

