While enforcement has been at a low ebb for quite some time with FDA’s Office of Prescription Drug Promotion (OPDP), this week took a different turn with the posting of two new untitled letters sent October 31. That brings the total number of letters issued by OPDP to five this year – one Warning Letter that came out in August respecting a sales aid used in promoting a treatment for COPD, and 4 untitled letters – one in June, one in August, and now two in October.
The first letter posted this week was issued on October 31 and was in sole relation to a direct-to-consumer brochure that contained promotional claims for an on demand contraceptive. In it, language was used to assign a specific percentage in relation to the product’s efficacy. FDA said that the use of this specific statistic was misleading in that it did not use a validated methodology and did not agree with the means used to describe efficacy in the PI for the product.
Likewise, the second letter posted this week, also issued on October 31, involved two different communications vehicles – a DTC broadcast advertisement and a banner ad that contained promotional language regarding an adjunct therapy for major depressive disorder – but focused on the same issue as the previous letter in relation to the promotional issue that concerned OPDP. Here again, OPDP compared the statistic used to describe efficacy with that of the PI for the product and found a difference between the two, resulting in the issuance of the untitled letter.
Looking for trends in recent enforcement, one does not see any pattern in terms of the types of communications vehicles – 2023 involved a Website, a sales aid, two banner ads, a brochure, and a DTC broadcast advertisement. And while contraceptives were involved in two of the 2023 enforcement actions, it seemed more like a coincidence than an area of concentration on the part of the agency. And they were pretty evenly divided between digital and traditional communications vehicles. There was no concentration among products with boxed warnings.
But there has been one trend worth noting. In fact, looking back to enforcement beyond this year through March of 2022, one can see that the last 7 letters issued by OPDP to pharmaceutical companies about promotional communications all involved a tie between a particular efficacy claim and the data from which it was derived, or where an efficacy claim appeared unsupported by data. So there is perhaps a message worth paying attention to here. The trend would indicate extra caution may be prudent when pharmaceutical and biotech companies are assigning specific performance percentages to their promotional claims when being derived from sources other than the PI for the product.
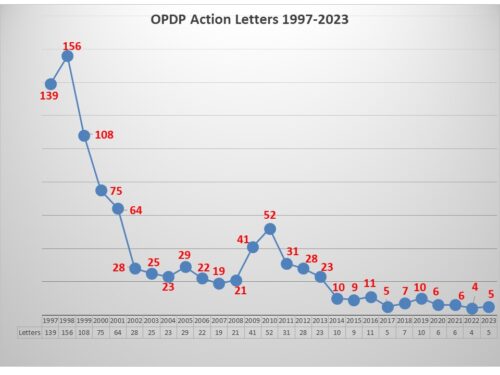
This leaves us with a total of five letters this year, more than last year, and less than the year before. In a period of such low enforcement it is difficult to discern a trend. But we still have several weeks left to the year, and in 2016, OPDP issued several letters in December to boost the total for that year into double digits. We’ll have to wait and see for 2023.

