
Each year Eye on FDA provides a look-back to see what the agency was talking about and what has changed over time. This year is a telling one and FDA may be changing the nature of the way they are communicating with the public. Moreover, this year is marked by the fact that the agency appeared to have less to say than in most recent years – perhaps taking a breather after the frenzy of the COVID-19 pandemic years.
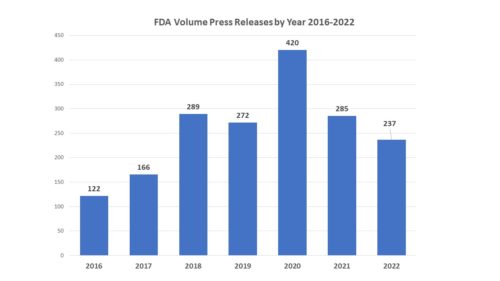
- Overall Fewer Press Releases – During 2022 the agency issued fewer press releases than in most previous years – 237 down from 285 the previous year and the fewest releases since 2017. There may be several factors at play. Not only was there less activity in relation to the COVID emergency, but we have had a change in leadership at FDA, which can have a big impact on how the agency communications. For example, when Dr. Gottlieb became FDA Commissioner he embraced the issuance of “Statements from the Commissioner” as a vehicle for talking about a vast array of topics, causing a huge spike in the number of releases from the agency. Prior to that time, such statements were very rare. With Dr. Califf at the helm, those statements have been used with far less frequency, issuing only a few this year. And in addition, there were far fewer new drug approvals in 2022, particularly new molecular entities, which means that there was less to talk about in that regard.
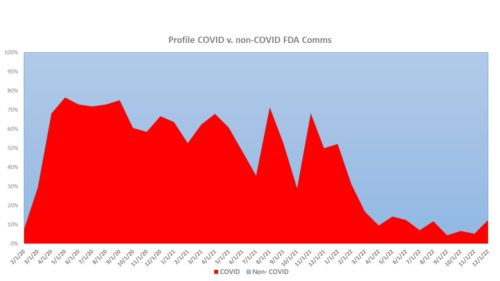
- The Air is Gone from the COVID Balloon – Needless to say, as the COVID-19 pandemic picked up pace, so did the agency’s involvement in approving new vaccines, new treatments and in addressing the thirst for reliable public health information. But the volume of COVID- related news has fallen off dramatically as has the agency’s activities. In 2020 there were 277 press releases related to COVID, with 144 in 2021, falling precipitously to only 27 in 2022. The chart below demonstrates the diminishing role on a month-by-month basis of COVID related releases (represented by the red area).
- FDA Began a New Method for Dispersing News – In prior years, the agency used to post news items that were not quite worthy of press release status in a vehicle called “FDA in Brief” that were posted in a place on the FDA website apart from traditional press releases. Beginning in 2022, however, FDA began to issue “FDA Roundups” which were included as part of the lineup of press releases, and “FDA in Brief” postings came to a halt. The new FDA Roundups contain a hodgepodge of news items rolled up into a single communication, topics which are not likely on their own worthy of a press release and which include mentions of COVID-related developments. They are generally issued usually twice a week, every third or fourth day, but not always. Unlike many of the agency releases, they are not translated into Spanish. This mechanism signals a whole new way for the agency to communicate its activities.
- What They Talked About – Finally, when they were talking, what were they talking about. As already noted, there were fewer approvals and less COVID-related material. There was a higher number of releases that were available in Spanish as well as English – and since there were fewer releases, that means that a higher proportion of releases were also in Spanish over years past.

While the number of press releases did diminish this year, given the added information going out in the regular roundups, the actual volume of information may increase. As we look forward to 2023, it may be important to note that since the month started 24 days ago, FDA has issued only 3 press releases in January, with the bulk of information being sent out via the Roundups.
Photo by AbsolutVision on Unsplash

