
For those who are regular followers of what FDA’s Office of Prescription Drug Promotion (OPDP) is up to, there was a surprise this week when the office posted the first regulatory action letter – in this case an Untitled Letter (a/k/a Notice of Violation (NOV) Letter) in over a year. The particulars of the letter are interesting, but first a quick review of recent enforcement from OPDP.
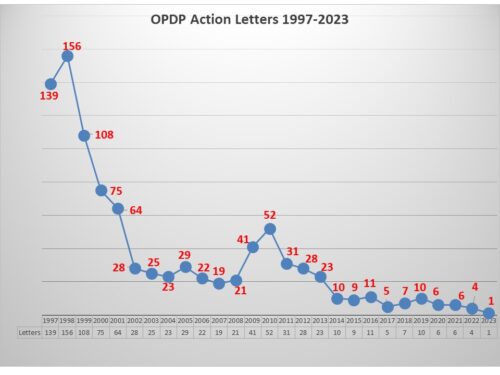
Enforcement Track Record – Looking all the way back to 1998, FDA issued a peak of 156 Untitled or Warning Letters in a single year (the prior year it had issued 139). That is the record high for a single year since that time and means that they were coming out a rate of over 12 a month on average. But the numbers began to dwindle and 2013 was the last time the office even issued 12 letters within a year (it did 23 that year). The numbers plummet in 2014 until the record low last year of only 4 letters. The last letter issued came just over a year ago in June, 2022.
Since then, crickets…..until this week.
The Newly Issued Letter – And now to the letter itself. True to form of late, this letter went to a company that is not a household name. It was the second letter for this company, the first having been issued in August, 2020 for a TV advertisement. The latest letter was issued for a website that promoted a treatment for hypercortisolemia in patients with Cushings Disease. The label for the medicine includes a boxed warning regarding potential issues related to complications for the liver (toxicity) and heart (QT prolongation).
OPDP took issue first with the description of efficacy which the agency said picked data points and reported efficacy of studies that, while accurate at that point in studies, did not reflect ultimate data points realized at the end of the final study. Further, the agency said, the product label reflected that a large number of patients discontinued treatment prematurely for multiple reasons and the omission of this information would impact the reader to assess efficacy.
On the risk side, the agency said the copy on the website that referred to monitoring and side effects failed to specifically outline the serious risks enumerated in the boxed warning, referring to them generally as serious side effects (rather than specifically calling out the boxed warning elements) and stating that testing will help a doctor avoid side effects. The agency felt that the lack of specificity combined with the implication that tests could help avoid adverse events minimized the risk realities given the seriousness of the potential adverse events and their frequency as outlined in the label. The agency acknowledged that full risk information was presented on the webpage, but felt that did not mitigate the overall characterization that minimized risk.
The ultimate lesson point in this action letter by OPDP is that when it comes to characterizing either efficacy or safety data, being as literal as possible in reflecting the label is important, particularly on the risk side when a product has a boxed warning.
A Note About Boxed Warnings – One might ask whether products with boxed warnings are more likely to face the possibility of an OPDP action letter. Going back and looking through 2020 at the products involved in letters, there have been a total of 17 letters (involving 19 different communications vehicles). Of those 17 letters issued, 8 had boxed warnings. Of those with 8 with boxed warnings, 5 of them were Warning Letters, as opposed to Untitled Letters. Of those without boxed warnings (9 in number), only 2 had Warning Letters. Not a huge pool from which to pull, but indicative nonetheless.
Back to Enforcement – At nearly the same time OPDP launched the “Bad Ad” program (2010) which is designed to deputize healthcare professionals and others to spotting and reporting potentially non-compliant communications, enforcement began to diminish. The agency holds trainings and gives continuing education credits. It forces the question why the agency would invest in such a program and then have fewer results to show for it? There are no apparent answers. It may be that the agency has focused resources on areas that present the highest risk and impact, and that this is not one of those areas.
Photo by Goh Rhy Yan at Unsplash

