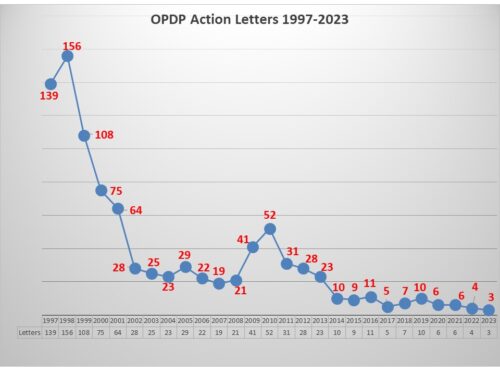
For the second time this month and the third time this year, FDA’s OPDP has posted notice that it has taken an enforcement action. Last week, a Warning Letter was posted regarding a sales aid. This week it action came in the form of an Untitled Letter (NOV) sent to a company regarding a paid social media posting. Enforcement this year was non-existent until June when the agency posted its first letter in a year. This week’s action makes a total of three letters sent this year, all within a three-month period.
The Untitled Letter posted this week involved an oral birth control pill which has a specific of contraindications contained in the label as well as a list of warnings and precautions and of the most common adverse events. The communications vehicle in question was a social media sponsored posting.
OPDP took issue with three specific aspects of the promotional communication. First, while presenting the indication in the posting, there was no risk information included in the communication. Second, a claim was made in the communication that OPDP said was not supported by the clinical studies section of the package insert. And third, the communication was not submitted under form FDA-2253 for review.
It would seem that the omission of any risk information combined with the review issue would have been enough to trigger action by OPDP. But as discussed last week, previous to this letter there were four letters in a row that held efficacy claims up against studies that might support the claims and found them wanting. This is now the fifth letter in a row that took issue with an efficacy claim with OPDP scrutiny of the underlying data that may or may not exist to support the claim. Pharma communicators take note.
Last year the agency only issued a total of four letters. Enforcement trends are impossible to predict but certainly it has picked up and there could be more to come. After all, back in 2016, the agency issued six letters in the month of December alone. Stay tuned.

