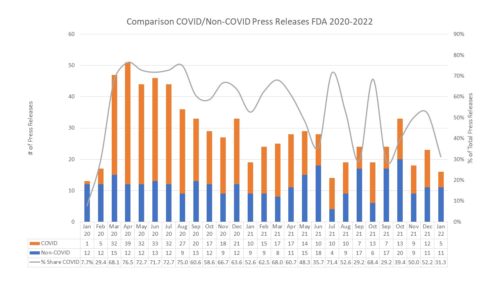
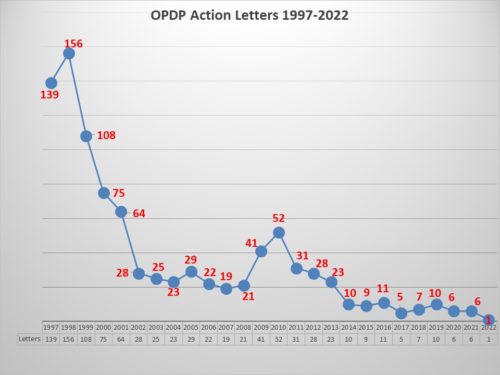
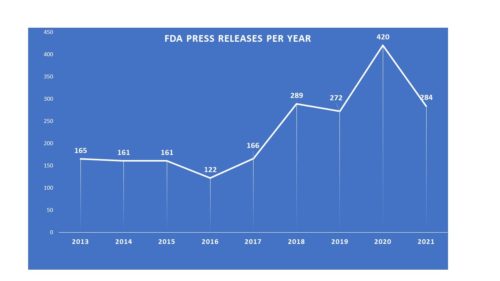
Tectonic pandemic plates are shifting respecting the COVID-19 pandemic. For weeks now we have been watching caseloads in the United States broadly fall. It feels as if we are indeed shifting gears. There are other moving parts that are in motion as well. It is a fundamental re-shifting of the landscape. And there are consequences.
First, government funding supporting access to vaccination and treatment for COVID has run out and so far, is not replenished. The government established program to fund access for the uninsured has stopped accepting reimbursement claims for treatment and will stop shortly for vaccines (on April 5). For the insured and affluent, not much of a problem. For those without, it can become a major roadblock. And if you need therapeutics, the same problem, but with harsher consequences, especially once the federally purchased stockpiles of therapeutics and vaccines are gone.
Second, next week an FDA Advisory Committee had scheduled a meeting for April 6 (the day after the government program will stop accepting claims for vaccines) to consider whether or not to authorize use of two of the COVID-19 vaccines as a “second booster” or actually a fourth shot, either for people over 50 years or for everyone. Then on March 29, FDA issued an authorization for use prior to the meeting of the advisory committee, quickly followed by a supporting update for providers to permit access from CDC. A fourth shot will likely be authorized by CDC, though access may be different than in the past, especially after the government stockpile recedes.
Third, the BA.2 sub variant 0f Omicron is poised to cause an increase in U.S. caseloads as it has in other parts of the world, thought it is not certain when. According to the Centers for Disease Control (CDC) it is now the dominant strain. After weeks of falling caseloads, some areas like New York City and New York State have seen the rates per 100,000 start to rise again and the 14-day change in cases has risen 92 and 78 percent, respectively as of yesterday, and have been increasing for the past several days. With the fourth booster in hand, those most vulnerable might opt to take it now (while there is stockpile) only to see it wane if the BA.2 wave in the U.S. does not happen until later in the year.
Fourth, the rise in availability of home testing is likely masking (pun intended) the actual numbers. Whole families may be find they are feeling unwell, testing and finding they are sick, but never getting sick enough to get medical attention. So they are completely under the radar when it comes to the numbers that we are counting and upon which we are basing policy decisions, like mask requirements.
Fifth, the masks are dropping. Attending a theatre performance this weekend I was double bagged – a KN95 underneath and a second (more fashionable) cloth mask over it. But I was in the minority by far. The lowering of mask requirements and inclinations may spur the emergence of the sub variant, or future variants.
Sixth, speaking of new variants, in places around the globe where there large numbers of people are being infected there is a potential fro the development of new variants.
In short, we are being dealt a new hand. A more spreadable virus is among us just as our vigilance is waning. More vaccine doses are authorized, but access is narrowed. Cases are increasing while mortality – a lagging indicator – has dropped. And perhaps most important, we are not assuring widespread access to vaccines and treatments to the most vulnerable, we are not accurately counting, and we are not taking precautions. From a policy perspective, perhaps we needed a new hand, but one thing remains – in the face of on-going infection, access to vaccines and therapeutics remains essential. While we are looking at a new landscape, we are also facing certain obstacles.
Photo by Casey Horner on Unsplash